In this article, we will discuss the atomic mass of an atom as well as understand the relationship between atomic mass and elemental mass. So starting from the atomic mass, which generally explains the relation of 1/12th part of a carbon-12 atom. This 1/12th part represents the one unit or one amu (atomic mass unit). Lets us understand it in a simple way, a carbon-12 atom has 6 protons and 6 neutrons in its nucleus. These nucleons suggested the atomic mass of C is 12 amu. Therefore, one proton unit simply represents one unit mass or 1 amu. So the atomic mass of any element is generally represented by its numbers of nucleons.
Example: Hydrogen has atomic number 1, which shows that H atom has only one proton in its nucleus; therefore, the atomic mass of hydrogen is 1 amu or 1 unit mass. Similarly, for lithium atom (At. No. 3) contains 3 protons and 4 neutrons in its nucleus show 7 unit of its atomic mass.
Now the question is, what will be the atomic mass of an element in grams?
This can also be calculated with the help of nucleons by taking the respective numbers of nucleons and their weight in grams. Let us understand the mass of an atom in grams.
Example: Hydrogen has one proton and no neutron; therefore, the mass of hydrogen atom would be
At this point, we know how to calculate the atomic mass of an atom in amu and in grams. Now let us talk about the term element. What are elements? In many textbooks, you will come across the definition of elements which says that "elements is the purest thing which can never be decomposed by any chemical or physical process". But what are elements actually and how they formed?
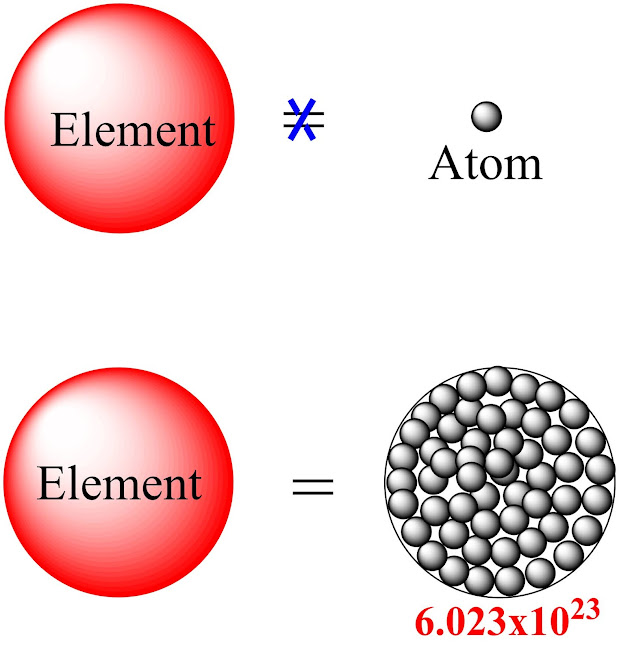
The elements are formed by the addition of a large number of atoms which gives the atomic weight in grams.
Weight of H element = Wt. of H atom x 6.023x10^23 g
6.023x10^23 also known as Avogadro numbers.
Now we understand that weight of elements comes from the combinations of a large number of atoms. So from the above equation one can also calculate the mass of one atom from their respective elements. Let us take some examples for ease of understanding.


Comments
Post a Comment